

Introduction: Basic Concepts of Solutions
a. Some Vocabulary
To prepare a solution by dissolution, you need to dissolve a solute in a solvent.
The solute is the substance dissolved in the solvent, and the solvent is the liquid in which the solute is dissolved.
If the solvent is water, the solution is aqueous.
Example:
- An aqueous solution of copper sulfate has copper sulfate as the solute and water as the solvent.
- If you mix iodine in alcohol, you get an iodine solution.
b. Mass Concentration
If I have 5g of salt in 1L of solution, I get a saltwater solution with a mass concentration (denoted Cm) of 5g/L.
And if I don't have a volume (denoted V) of 1 liter? → Cm represents the ratio between m and V, so the relationship is:
- Cm = m / V or m = Cm x V
- Cm is the mass concentration in g/L.
- V is the volume of the solution (not the solvent!) in liters (L).
- m is the mass of solute in grams (g).
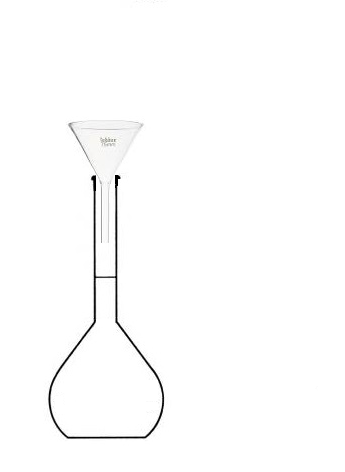
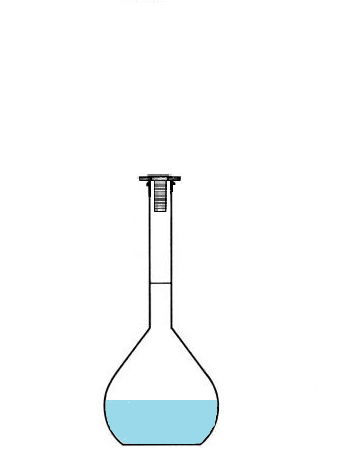
c. Preparing a Solution with a Volumetric Flask?
If I mix 10g of salt and 100mL of water, I get a solution with a volume of 100mL.
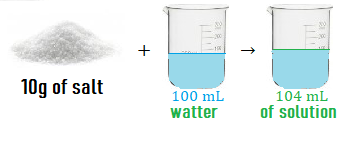
You are right 😎, even dissolved, the 10g of salt have a volume.
We get a solution volume greater than 100 mL.
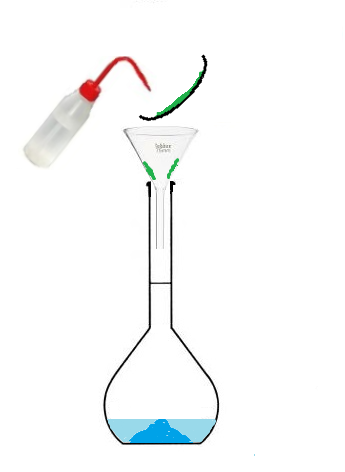
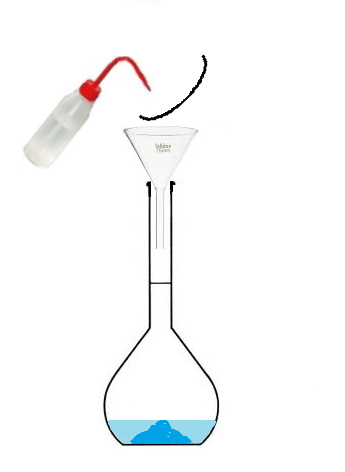
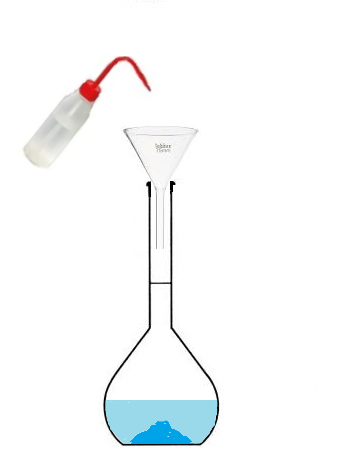
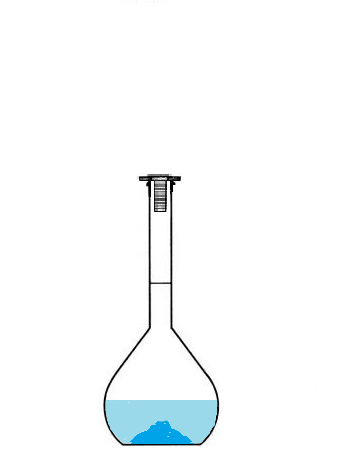
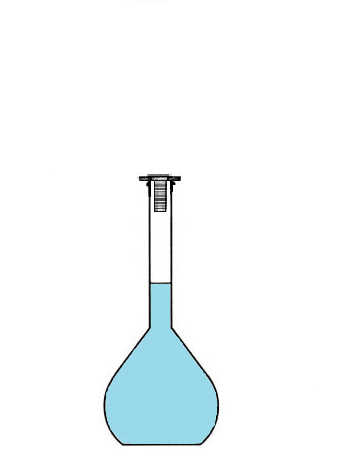
Conclusion: To prepare a solution of volume 100 mL, we will not use 100mL of solvent
but we will use a volumetric flask that we will fill up to the mark.
We get a solution volume greater than 100 mL.
Conclusion: To prepare a solution of volume 100 mL, we will not use 100mL of solvent
but we will use a volumetric flask that we will fill up to the mark.

Which volumetric flask should you choose if you want a solution of volume 150 mL?
You should choose a 250 mL flask, then take a volume of 150mL,
with, for example, a graduated cylinder.
The volume of prepared solution corresponds to the volume of the volumetric flask,
since we will fill it up to the mark.
with, for example, a graduated cylinder.
The volume of prepared solution corresponds to the volume of the volumetric flask,
since we will fill it up to the mark.
d. Knowing How to Prepare a Solution by Dissolution
We offer you a simulation, in which you will prepare a copper sulfate solution (with random concentration).
Here are the steps:
- Weigh the copper sulfate powder in a weighing boat on a balance.
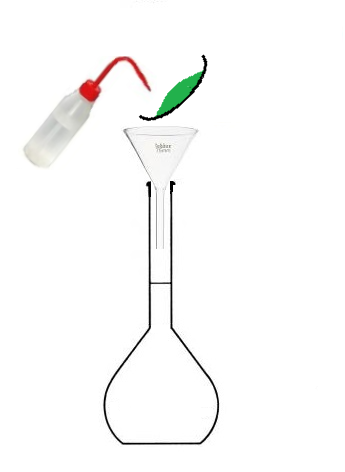
- Pour the copper sulfate into a beaker of water.
- Pour the beaker into the volumetric flask,
- then fill with water up to the mark.
1. Calculate the mass of solute to weigh
We want to prepare a solution with a volume of mL and a mass concentration of g/L
Let's recall the formula given in the introduction: Cm = m / V or m = Cm x V
Using the SuperCalculator 😁, find the mass of solute to dissolve (the mass should appear in the purple box).
SuperCalculator 😁
x
=
🔼 drag / drop 🔼
We want to prepare a solution with a volume of mL and a mass concentration of g/L
Click on the equipment you need (click again to remove selection):
✔️: selected ❌: not selected
✔️: selected ❌: not selected

25 mL graduated cylinder
❌

100 mL graduated cylinder
❌

50 mL volumetric flask
❌

100 mL volumetric flask
❌

500 mL volumetric flask
❌

1000 mL volumetric flask
❌

electronic balance
❌

dropper pipette
❌

anhydrous copper sulfate
(anhydrous: without water)
(anhydrous: without water)
❌

10 mL volumetric pipette
❌

watch glass
❌

funnel
❌

pipette filler
❌

wash bottle
❌
We want to prepare a solution with a volume of mL and a mass concentration of g/L
In this part, we will simulate the weighing of the solute that was calculated in step 1.
Therefore, the balance should display a mass of g.
We want to prepare a solution with a volume of mL and a mass concentration of g/L
In this simulation, you will prepare the solution