Redox Reactions
Based on the observations of the biologist Galvani, the Italian physicist Volta conducted research that led him to establish his "series of metal tensions" in 1793.In 1800, he discovered the electric battery that bears his name. These discoveries laid the foundation for the interpretation of redox phenomena.
I. First Concept of Redox Reactions
1. Can a metal react with a metallic ion?
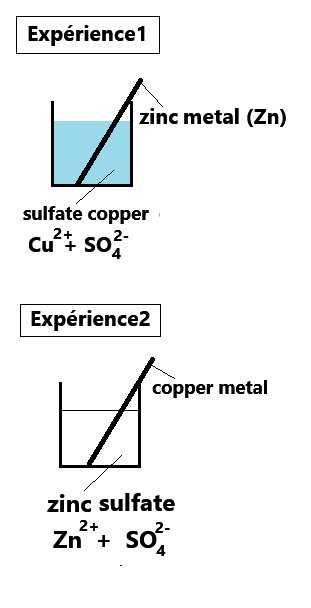
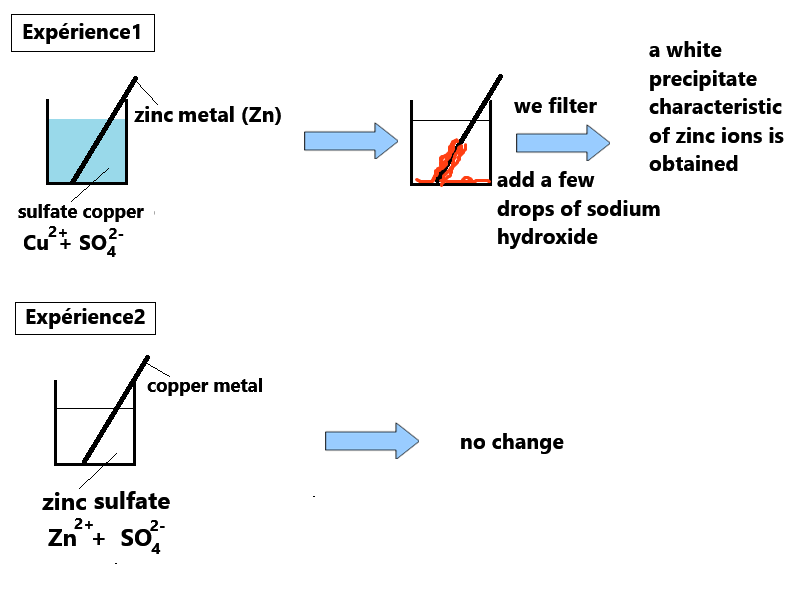
Observations for experiment 1:
Reminder: The blue color is characteristic of Cu2+ ions in water.
Now, it's your turn to interpret experiment 1 by selecting the 10 correct answers below!
- The blue coloration of the solution is significantly reduced
- A red-orange deposit, characteristic of metallic copper (Cu), is observed
- A white precipitate, characteristic of Zn2+ ions, is formed
- No change, indicating that no chemical transformation occurred
Reminder: The blue color is characteristic of Cu2+ ions in water.
Now, it's your turn to interpret experiment 1 by selecting the 10 correct answers below!
Correct answer(s): 0/10Errors: 0
Congratulations! You found all the correct answers!
2. Writing the Reaction Equation
We proceed step by step:
Zn → Zn2+ + 2e- (Oxidation: Zn loses 2 electrons) Cu2+ + Zn + 2e-❌→ Cu + Zn2+ + 2e-❌ (Overall redox equation)
Cu2+ + Zn → Cu + Zn2+ (Overall redox equation)
Note: The electrons cancel out directly. If this is not the case,
one of the two half-equations must be multiplied by a coefficient to balance the electrons.
- We write two half-equations that correspond to the loss or gain of one or more electrons.
- We sum these two half-equations to obtain the overall reaction equation.
Zn → Zn2+ + 2e- (Oxidation: Zn loses 2 electrons) Cu2+ + Zn + 2e-❌→ Cu + Zn2+ + 2e-❌ (Overall redox equation)
Cu2+ + Zn → Cu + Zn2+ (Overall redox equation)
Note: The electrons cancel out directly. If this is not the case,
one of the two half-equations must be multiplied by a coefficient to balance the electrons.
- In experiment 1, the electrons lost by Zn are captured by Cu2+.
- The oxidant Cu2+ is transformed into Cu, which is a reductant.
- Thus, we have the redox pair Cu2+ / Cu.
- Similarly, we have the redox pair Zn2+ / Zn.
- A redox pair is always written in the form oxidant / reductant.
II. In General
1. Basic Concepts
- A chemical species capable of donating one or more electrons is a reductant.
- A chemical species capable of accepting one or more electrons is an oxidant.
- During a redox reaction, the oxidant of one pair is reduced, and the reductant of another pair is oxidized.
2. A Rule: Can We Predict the Spontaneous Reaction Between Two Redox Pairs?
Experiment 2 shows that simply combining an oxidant and a reductant does not necessarily lead to a chemical reaction.
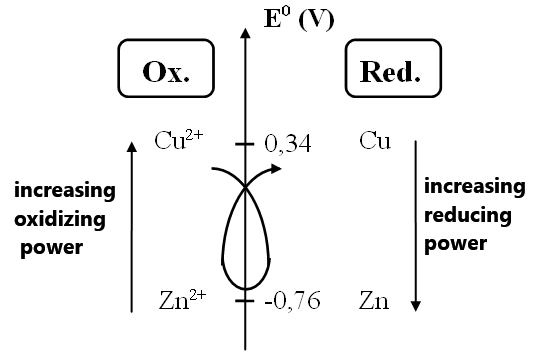

Volta's work led to the association of a standard redox potential E0 (in Volts (V)) with each oxidant/reductant pair.
Using these values, it was possible to rank all the pairs.
Using these values, it was possible to rank all the pairs.
- The higher the E0 value, the stronger the oxidant in the pair and the weaker the reductant.
- Cu2+ is therefore a stronger oxidant than Zn2+.
- Zn is a stronger reductant than Cu.
- The spontaneous redox reaction between two pairs always occurs between the strongest reductant and the strongest oxidant
(this is known as the "gamma rule"). - This explains why Cu2+ and Zn react, whereas Zn2+ and Cu do not.
3. Synthesis Exercise
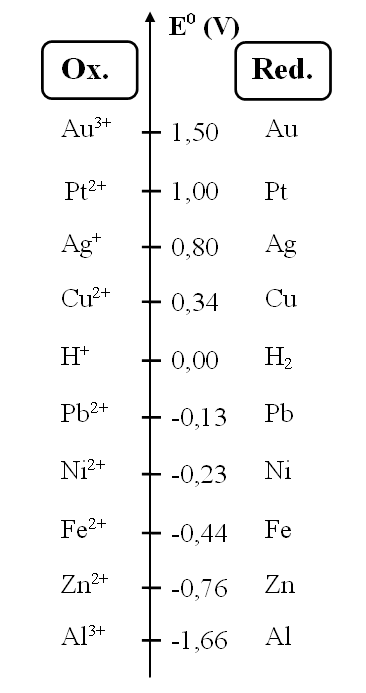
a. Select the 3 metals that oxidize the least easily
Congratulations, you found it!
The highest potential corresponds to the weakest reductant, meaning the one that reacts the least.
b. Indicate whether a reaction is possible between:
c. Drag and drop to obtain the reaction equation between Ag+ and Fe
Congratulations, you found the correct solution
🔽 drag & drop 🔽
( → + e-)x
( + e- → )x
Fe + 2Ag+ → Fe2+ + 2Ag
III. Other Important Oxidant/Reductant Pairs
There are other redox reactions where metals do not take part. Acidified aqueous solutions are often used.
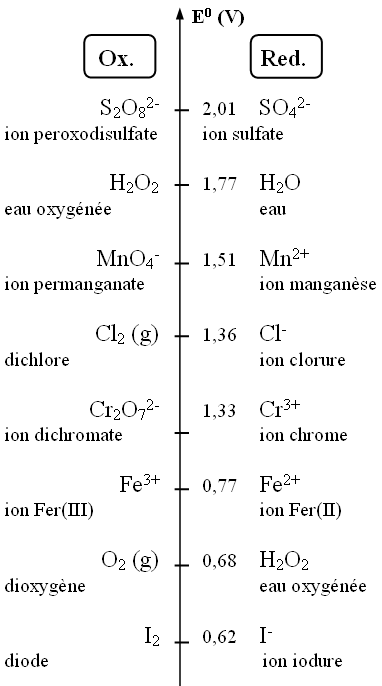
- H2O is present in the solvent
- If acid is added, H+ is also present as it is responsible for the acidic nature of the solution
- H2O and H+ can therefore take part in the reaction equations

a. Drag and drop to obtain the possible reaction between the pairs I2 / I- and S2O82- / SO4-
Well done, you found the correct solution
🔽 drag / drop 🔽
( + e- → )x
( → + e-)x
S2O82- + 2I- → 2SO4- + I2
b. Drag and drop to obtain the possible reaction between the pairs MnO4- / Mn2+ and O2 / H2O2
Well done, you found the correct solution
🔽 glisser / déposser 🔽
(
+ e-
+
→
+
)x
(
→
+
+
e-)x
10e-❌ + 2MnO4- + 16H+❌6H+ + 5H2O2 → 2Mn2+ + 8H2O + 5O2 + 10H+❌ +10e-❌
2MnO4- + 6H+ + 5H2O2 → 2Mn2+ + 8H2O + 5O2