Relation between Mass and Molar Mass
a. Counting by Mole
A drop of water contains billions of billions ... of water molecules
To express the quantity of substance in chemistry, we use a quantity that contains many elements:
- In chemistry, we count elements in moles (mol for short)
- 1 mole contains 6.02x1023 entities
- The quantity of substance is thus expressed in moles with the symbol n
- This number 6.02x1023, is called Avogadro's number noted NA
Examples:
- n(H2O) = 1 mole corresponds to 6.02x1023 water molecules
- n(H2O) = 0.5 mole corresponds to 0.5x6.02x1023 water molecules, i.e., 3.01x1023 molecules.
If N is the number of elements counted per unit: N = n x NA
Note: in practice, we often use n without necessarily needing N,
but it is important to know that n represents a number of "packets".
b. From Mole to Mass
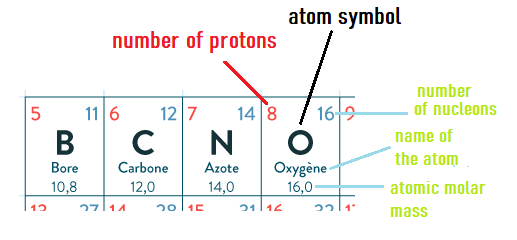
If we consider one of the chemical elements, such as oxygen(O), and weigh 1 mole of oxygen atoms,
we obtain what is called the atomic molar mass of oxygen.
This is a reference value found in the periodic table, an excerpt of which is shown below:
It is impossible to list the molar masses of molecules (referred to as molecular molar mass),
there are far too many.
- A molecule is an "assembly" of atoms
-
The molecular molar mass is therefore equal to the sum
of the atomic molar masses of all the atoms that compose the molecule.
For example:
- We know that MH = 1.0 g/mol and MO = 16.0 g/mol
- So M(H2O) = 2 x MH + 1xMO
- M(H2O) = 2x1 + 1x16 = 18 g/mol
Let's check if I understood correctly:
We can deduce that MC2H6O = 46.0 g/mol
c. From Molar Mass to Mass.

For this sugar cube, I can determine the following quantities:
- n → quantity of substance in mol
- M → molar mass in g/mol
- m → mass in g
For 1 mole → m = M (by definition M represents the mass of one mole)
For n moles → m = n x M or n = m/M.
Let's summarize what we've just seen with the example below:
- If we weigh a sugar cube, we find approximately 6g
- The molecule composing sugar is sucrose with the formula C12H22O11
The molar mass of sugar is MC12H22O11 = 342g/mol and the quantity of substance in a sugar cube (6g) is n = 0.25 mol.
Now, it's your turn to play! A mass or quantity of substance (random) to find.
Which compound would you prefer to work on?:
Relation between Mass, Molar Mass, and Quantity of Substance



The molar mass should appear on the violet box
of SuperCalculator 😁.