


Dilutions
We have already studied mass concentration here. As a reminder, it is denoted Cm and represents the ratio between the mass of solute and the volume of solution.
This corresponds to the "proportional" formula: Cm = m/V
Similarly, if we look at the amount of substance rather than the mass, we can define the molar concentration denoted as C.
The molar concentration represents the ratio of the amount of solute to the volume of solution, which leads to the formula C = n/V.
- C: molar concentration in (mol/L)
- n: amount of solute in moles (mol)
- V: volume of solution in liters (L)
Quantities and Units:
In fact, C and Cm are two quantities that are also proportional to each other: Cm = C x M or Cm/C = M, where:
- M: molar mass of the solute in (g/mol)
- Demonstration: Cm = m/V, but m = n x M (seen here), so Cm = (n x M)/V = (n/V) x M = C x M (since n/V = C)
Conclusion: It is logical that the amount and the mass are proportional. You must be able to use both C and Cm and switch from one to the other easily!
A practical case: To cook my pasta, I prepare a solution of saltwater (or an aqueous solution of sodium chloride!).
- Data:
- Salt formula: NaCl
- Solution volume: V = mL, salt mass: m(NaCl) = g
- Molar masses:
- M(Na) = 23.0 g/mol
- M(Cl) = 35.4 g/mol
Your mission, should you choose to accept it, will be to:
- Display the mass concentration of the salt in a green box of the SuperCalculator 😁
- Display the molar concentration of the salt in a purple box of the SuperCalculator 😁
If you are lost, click on
1. What is a dilution?
Let’s take the example of a saltwater solution (solute: salt, solvent: water), it's "too salty," and I want to dilute it.
To dilute means to obtain a solution with less salt concentration,
... So to dilute, you will add water! Because diluting is simply adding solvent (water for aqueous solutions).
2. Vocabulary and notations
The initial solution (the more concentrated one) is called the mother solution.
The diluted solution (less concentrated) is called the daughter solution.
-
Notations:
- Subscript 0 for the mother solution and subscript f for the daughter solution
- Vf: volume of the daughter solution, V0: volume of the mother solution
- Cf: molar concentration of the daughter solution, C0: molar concentration of the mother solution
3. The dilution formula
In 1g of salt, there are approximately 1022 ions; the human brain is not "wired" to visualize such a large number.
To better understand, imagine a solution of smileys! It contains a small number of smileys, and let’s perform a dilution:

Something stays the same after the dilution,
... it’s the number of smileys. It’s logical because when you dilute,
you add solvent but no solute, so the amount of substance and the mass of solute do not change.
This simple observation leads to the dilution formula:
- For the mother solution:
- The amount of substance n0 = C0 x V0
- For the daughter solution:
- The amount of substance nf = Cf x Vf
- Since the amount of solute does not change:
- n0 = nf, therefore C0 x V0 = Cf x Vf
This is the dilution formula. In part 3, you will learn how to find the literal expression of each quantity!
-
Important notes:
- V0 and Vf must have the same unit (not necessarily liters (L); both volumes can be expressed in milliliters (mL), for example).
- Similarly, both concentrations C0 and Cf must have the same unit.
- C0 and Cf can also be mass concentrations.
Introduction
In the laboratory, we have a mother solution with concentration C0 .
We want to create a less concentrated solution by dilution with concentration Cf and volume Vf .
Data:
- Molar concentration of the mother solution: C0 = mol/L
- Molar concentration of the daughter solution: Cf = mol/L
- Volume of the daughter solution: Vf = mL
Remarks:
- The methodology and calculations do not depend on the nature of the solution.
- However, proper safety practices must be followed depending on the solution (gloves, goggles, working under a fume hood).
- The values for C0, Cf, and Vf are randomly generated.
Starting from the mother solution with concentration C0, your mission is to create a daughter solution with concentration Cf and volume Vf.
The steps:
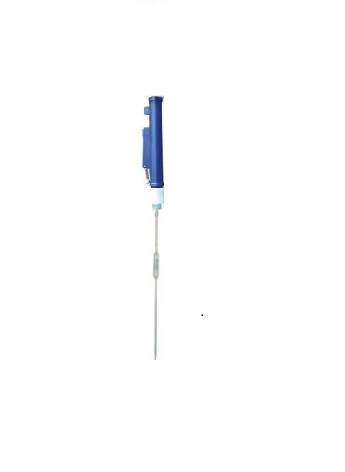
- a. Calculate the volume of the mother solution to be taken
- b. Make a list of equipment needed to perform the dilution
- c. Perform the dilution
Data:
- Molar concentration of the mother solution: C0 = mol/L
- Molar concentration of the daughter solution: Cf = mol/L
- Volume of the daughter solution: Vf = mL
- Dilution formula: C0 x V0= Cf x Vf
In this section, your mission is to calculate the volume of the mother solution to take (V0) to prepare the daughter solution.
The value of V0 (in mL) should appear in the violet box of the SuperCalculator 😁.
If you're stuck, click
We want to prepare a solution with volume Vf = mL and molar concentration Cf = mol/L,
by taking a volume V0 = mL of the mother solution.
✔️: selected ❌: not selected

















We want to prepare a daughter solution with volume Vf = mL by taking a volume V0 = mL of the mother solution.
In this simulation, you will prepare the solution
Progression : 0 / 4
- C0 =
- V0 = ...
- Cf = ...
- Vf = ...