Click on the correct color
negatively charged ion
atom (no charge)
positively charged ion
cards flipped: 0/18
Click on the correct color
cards flipped: 0/18
The Atom ⚛️

Your mission is to find the number of outer electrons of the chemical element below:
- To help you:
- la règle de Klechkowski: 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f
- you can use the superCalculator😁 which allows you to add the electrons of the saturated subshells
Core symbol:
126CElement has electrons
Electronic distribution gives:
The outer layer is the
We therefore have external electron(s).
So you have
1. What do we do?
- Electronic Configuration
- Electronic Structure
- Electron Cloud
- Electronic Formula
Here, we will only focus on learning how to distribute electrons across electron shells and subshells.
To do this, we’ll use Bohr’s planetary model (outdated) and Klechkowski’s rule.
While representing electrons in circular orbits is incorrect, it helps simplify the understanding of how electrons are distributed.
2. Shell and subshell distribution
The terms "shells" and "subshells" come from Bohr’s model, which described electrons as orbiting in circular paths.We’ll proceed step by step: a) shells → b) subshells → c) I have more than 18 electrons to distribute.
a. Distributing electrons across electron shells
- Logically, we first place an electron in a shell and then in a subshell.
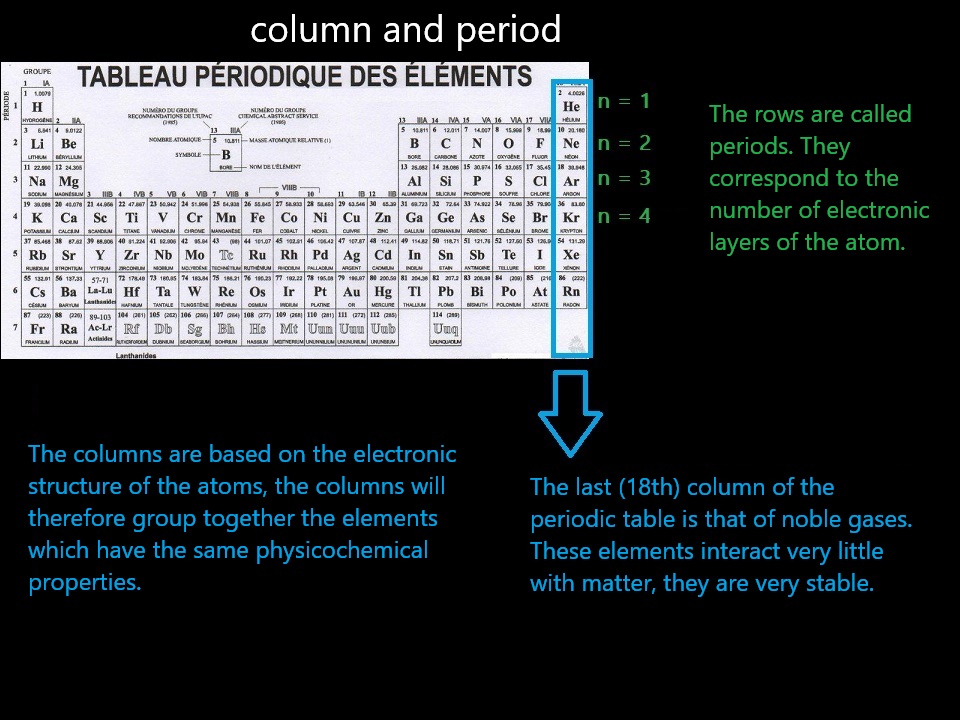
- Each shell is characterized by an integer (n > 0) (starting from 1!).
- A shell can hold up to 2n² electrons (e.g., the 3rd shell (n = 3) holds 2 × 3² = 18 electrons).
- (n = 1) → 1st shell, (n = 2) → 2nd shell, and so on...
- If we have 18 or fewer electrons to distribute, we must fill the previous shells before moving to the next one.
- The shell number is written with the number of electrons in superscript: (3²) means "I have 2 electrons in the 3rd shell".
- 1st shell: 2n² = 2 × 1² = 2 electrons → 12 (remaining: 12 - 2 = 10 electrons)
- 2nd shell: 2n² = 2 × 2² = 8 electrons → 12 28 (remaining: 10 - 8 = 2 electrons)
- 3rd shell: 2n² = 2 × 3² = 18 electrons → 12 28 32, but only 2 electrons remain for this shell.
b. Distributing electrons across subshells
- Each subshell is characterized by an integer l such that 0 ≤ l < n (it starts from 0 and goes up to n - 1).
- For example:
- (n = 1) → (l = 0)
- (n = 2) → (l) can be 0 or 1
- (n = 3) → (l) can be 0, 1, or 2...
- Each subshell corresponds to a letter:
- (l = 0) → s (sharp)
- (l = 1) → p (principal)
- (l = 2) → d (diffuse)
- (l = 3) → f (fine), etc.
- Each subshell can hold 2(2l + 1) electrons. We must fill each subshell before moving to the next.
- s → (l = 0) → 2 electrons
- p → (l = 1) → 6 electrons
- d → (l = 2) → 10 electrons
- f → (l = 3) → 14 electrons
- Now, let’s apply the rules to distribute 12 electrons:
- 12 → 2 electrons in the s subshell → 1s2
- 28 → 2 electrons in 2s, 6 in 2p → 2s2 2p6
- 32 → 2 electrons in 3s → 3s2
- We fill the first two shells the same way: 1s2 2s2 2p6. That leaves 18 - 10 = 8 electrons to distribute in the 3rd shell.
- 38 → 2 electrons go into the s-subshell → 3s2, 6 electrons go into the p-subshell → 3p6, so for the 3rd shell we get 3s2 3p6.
- Finally, we have: 1s2 2s2 2p6 3s2 3p6.
c. Distributing more than 18 electrons
Once we exceed 18 electrons, the rule about filling all previous shells before moving to the next one no longer holds. Instead, we apply Klechkowski's rule:
You can see that after filling 3p (3p6), we move to 4s, and after filling this shell (4s2), we return to the 3rd shell to fill 3d, and so on.
d. The outer shell
1. Particles
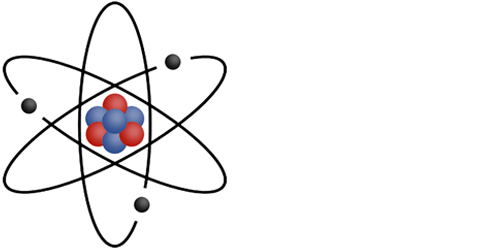
This representation is not experimentally accurate,
but it helps easily identify the particles that make up an atom.
-
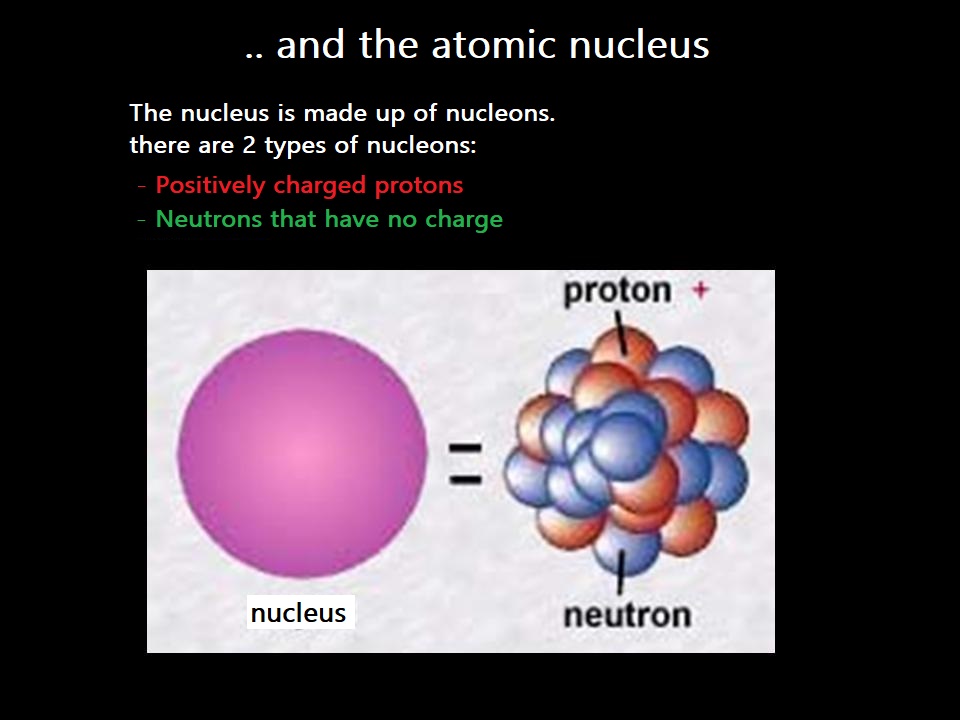
The nucleus (at the center) is composed of two different particles:
- Protons (in red)
- Neutrons (in blue)
- Electrons (in black) moving around the nucleus
A nucleon is a particle of the nucleus, so it can be either a neutron or a proton.
2. Key Values of Particles
| Name | Mass (kg) | Electric charge (C) |
|---|---|---|
| Electron | melectron = 9.109 × 10-31 kg | -e = 1.602 × 10-19 C |
| Proton | mproton = 1.673 × 10-27 kg | +e = 1.602 × 10-19 C |
| Neutron | mneutron = 1.675 × 10-27 kg | 0 |
- The unit of electric charge is the Coulomb (C).
- e is the elementary charge, and you’ve noticed that protons and electrons carry opposite charges!
3. Using Key Values: Think and Click!
- Gaining electrons → The ion is negatively charged (anion), e.g., fluoride ion F- (gained one electron).
- Losing electrons → The ion is positively charged (cation), e.g., copper(II) ion Cu2+ (lost two electrons).
- The nucleus is made of neutrons (charge 0) and protons (charge +e).
- Therefore, the nucleus is positively charged!
- A nucleus without protons (and thus no charge) doesn’t exist. The smallest atom, hydrogen, has one proton in its nucleus.
If we use 3 significant figures instead of 4, we get the same value: 1.67 × 10-27 kg for both.
Therefore, we can say that the two masses are practically identical, and the mass of a nucleon is mnucleon = 1.67 × 10-27 kg.
However, be cautious about rounding values too often, especially in microscopic contexts!
If we roughly calculate the ratio between mnucleon and melectron, we get:
mnucleon / melectron ≈ 2000.
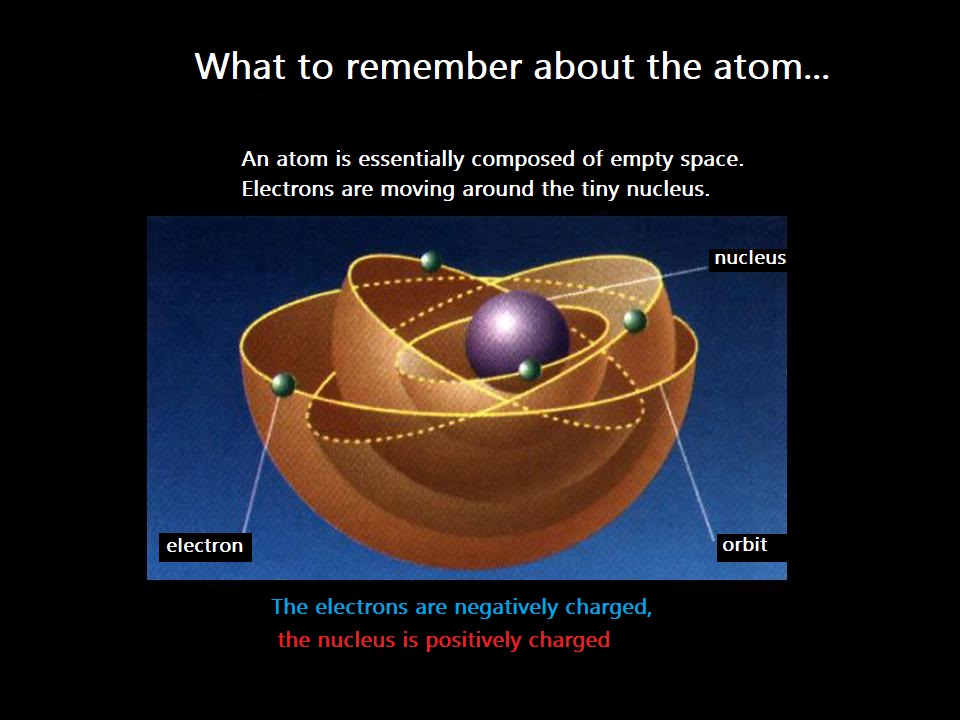
This means that the mass of electrons can often be neglected compared to nucleons. In other words, the mass of the atom is concentrated in its nucleus.
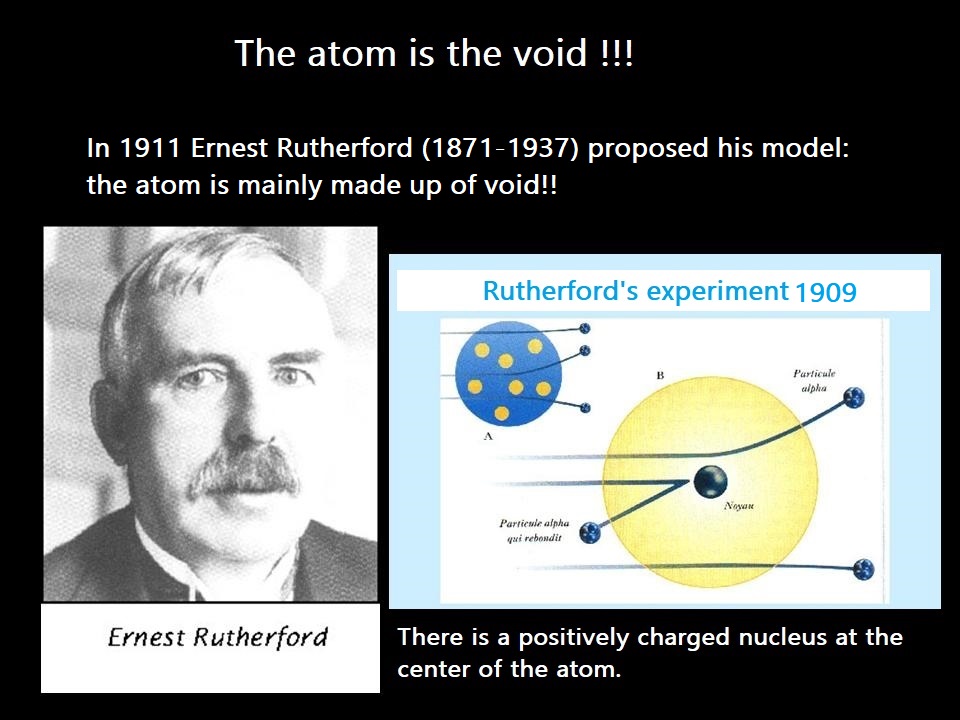
Moreover, the atom’s diameter is 100,000 times larger than the diameter of its nucleus!
The atom is mostly empty space!
Rutherford demonstrated this with the famous "gold foil" experiment in 1909.
Since protons and electrons carry opposite charges, let’s take Carbon as an example:
Carbon has 6 protons and 6 electrons, so the overall charge is +6e - 6e = 0. Therefore, like all atoms, Carbon is electrically neutral.
If an atom gains or loses electrons, it becomes an ion. Since electrons carry a negative charge:
4. Nucleus Symbol
- X: Chemical element symbol
- A: Number of nucleons
- Z: Number of protons
- Example of a carbon atom: 126C
- A = 12, so carbon has 12 nucleons.
- Z = 6, so carbon has 6 protons.
- An atom also has Z electrons, so carbon has 6 electrons.
- N = A - Z = 12 - 6 = 6, so carbon has 6 neutrons.
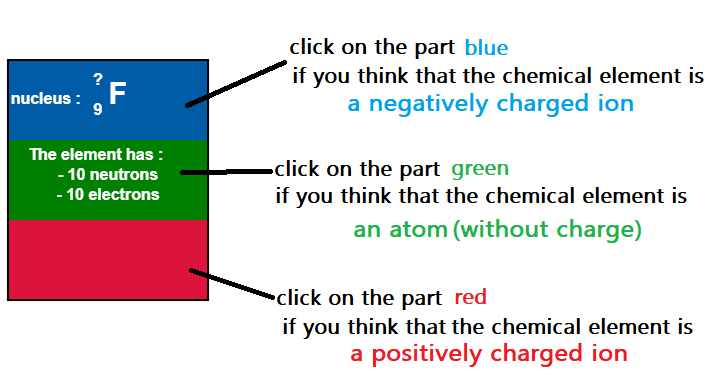
- The negative charge indicates the ion's overall charge (the nucleus remains positively charged).
- 9 protons (Z = 9)
- 19 nucleons (A = 19)
- 10 neutrons (N = A - Z = 19 - 9 = 10)
- 10 electrons (9 original electrons + 1 extra).
5. Isotopes and Chemical Elements
Have you ever heard of Carbon-14 or Uranium-235? What do these numbers mean?
Well, these numbers help identify the atom, because Carbon-12 or Uranium-238 also exist!
More precisely, these numbers correspond to the number of nucleons. So, Carbon-12 and Carbon-14 have different nuclei. But why do they have the same name then?
- Here are three different carbon nuclei: 126C
136C
146C .
- A different number of nucleons (12, 13, 14), and thus a different number of neutrons (6, 7, 8).
- Same symbol (C), same name (Carbon), and ... the same number of protons (6)!
A chemical element is defined by the number of protons (Z) in its nucleus.
Isotopes are "variants" of the same chemical element. They all have the same number of protons but a different number of neutrons (or nucleons).
- Carbon-12 has N = A - Z = 12 - 6 = 6 neutrons.
- Carbon-13 has N = A - Z = 13 - 6 = 7 neutrons.
- Carbon-13 has one extra neutron, so it is slightly heavier.
- Avogadro’s number would be smaller (fewer atoms would be needed to reach 12g).
It is the number of carbon atoms in 12g of Carbon-12.
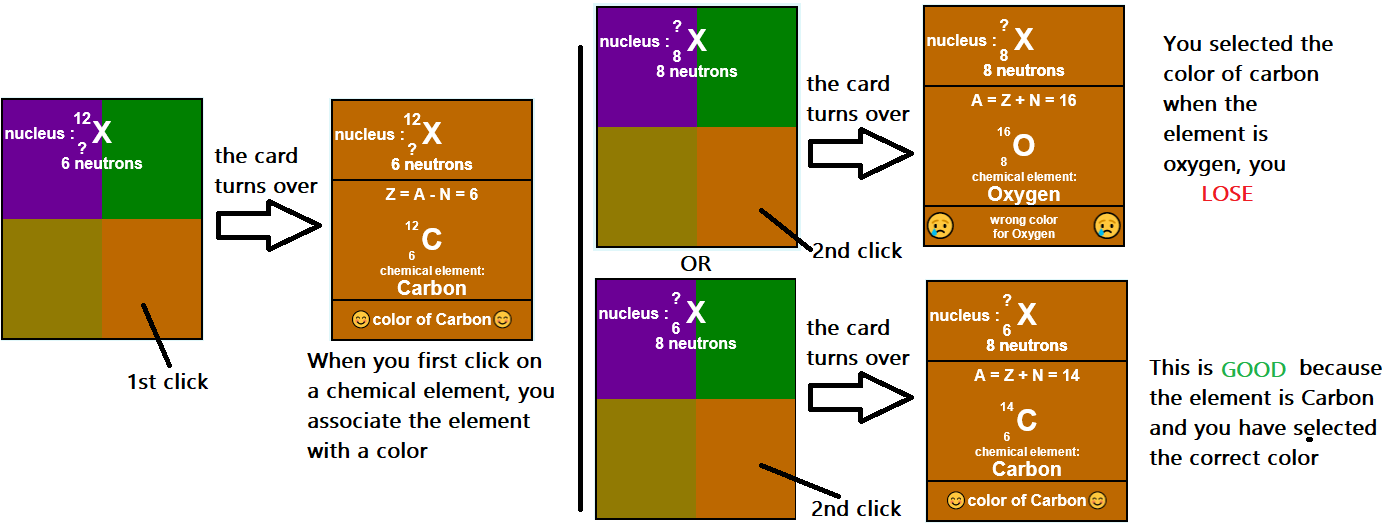
The Rules of the Game: They Are Very Simple!
18 cards are laid out in front of you. For each card, you just need to click on the correct color. That’s all...
One small detail... You lose if you make a mistake! So, to finish the game, you need to flip all 18 cards without making any errors 😁.
The Rules of the Game: They Are Very Simple!
18 cards are laid out in front of you. For each card, you need to click on the correct color. That’s all...
- A chemical element (e.g., Carbon) is associated with the color you chose at the beginning.
- One color is linked to one chemical element.
- You win the game if you flip all 18 cards without making a mistake.
-
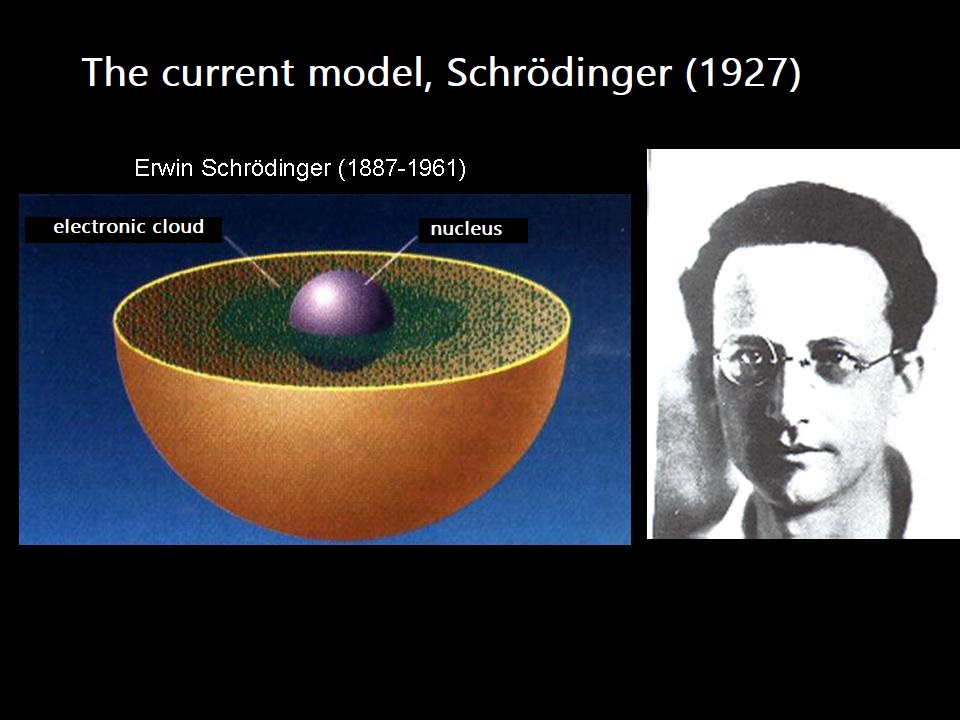
First, it is a representation:
- But (hopefully), no one looks like this! A scientific model must be consistent with reality.
-
Here, "consistent" does not mean "equal," but it should come as close as possible. To achieve this, certain criteria must be met:
- A scientific model is validated by experiment (one experiment alone can prove it wrong).
-
It is based on hypotheses that must be clearly stated.
-
For example:
- Light travels in a straight line.
-
When the medium is opaque, it is reflected and/or absorbed.
When light changes medium (e.g., when it passes from air to water — refraction phenomenon), it is deflected.
In a homogeneous and transparent medium, light travels in a straight line. The missing hypotheses make all the difference!
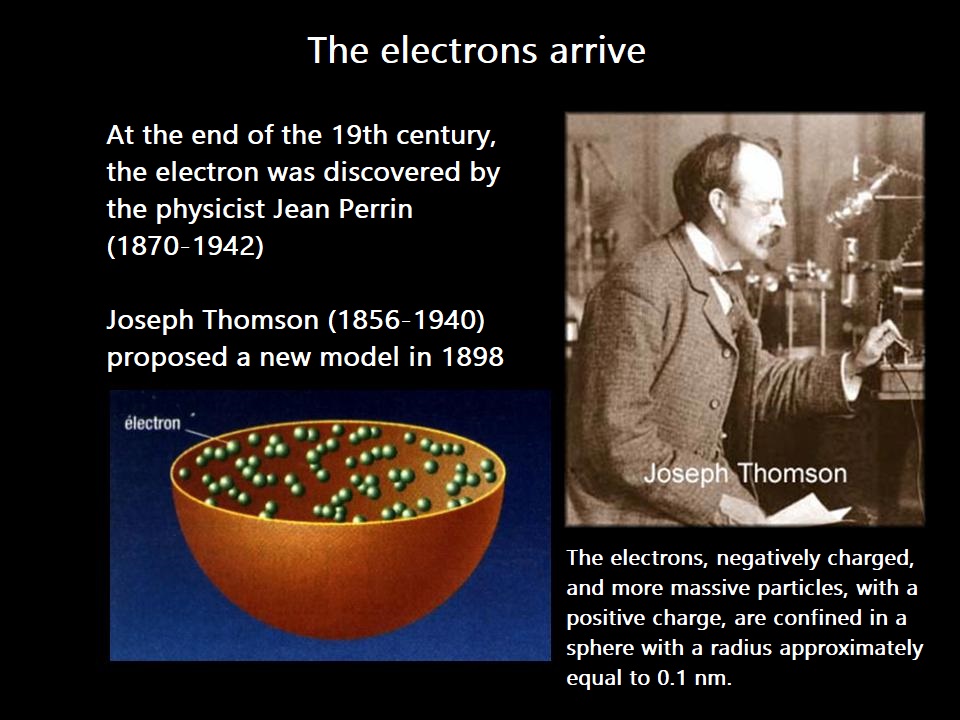
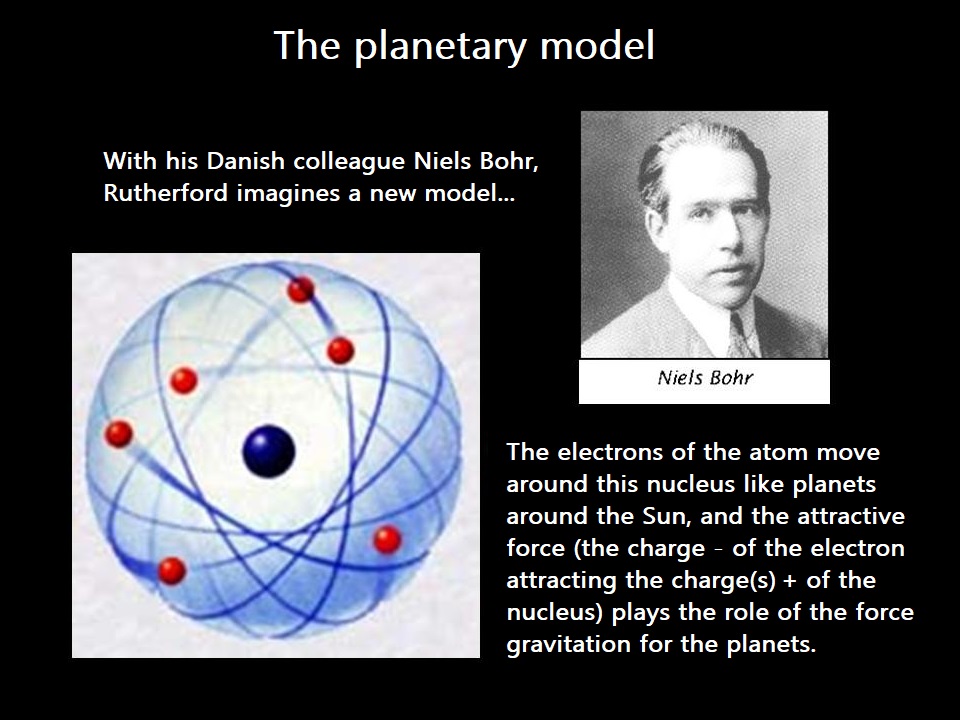
A scientific model evolves throughout history and also through our learning!
When I address electronic structure, I will use Bohr's planetary model, for example.
Even though this model is not consistent with experiments, the concepts introduced will be easier to understand compared to the probabilistic model.
Your mission is to find the stable ion produced from the atom .
- To help you:
- The ion copies the electron structure of the noble gas it is closest to.
- So it has the same number of electrons...
- Symbols of the nuclei (noble gases): 2He, 10Ne, 18Ar, 36Kr, 54Xe
Adjust the number of electrons, then click on